What Is The Ozone Hole?
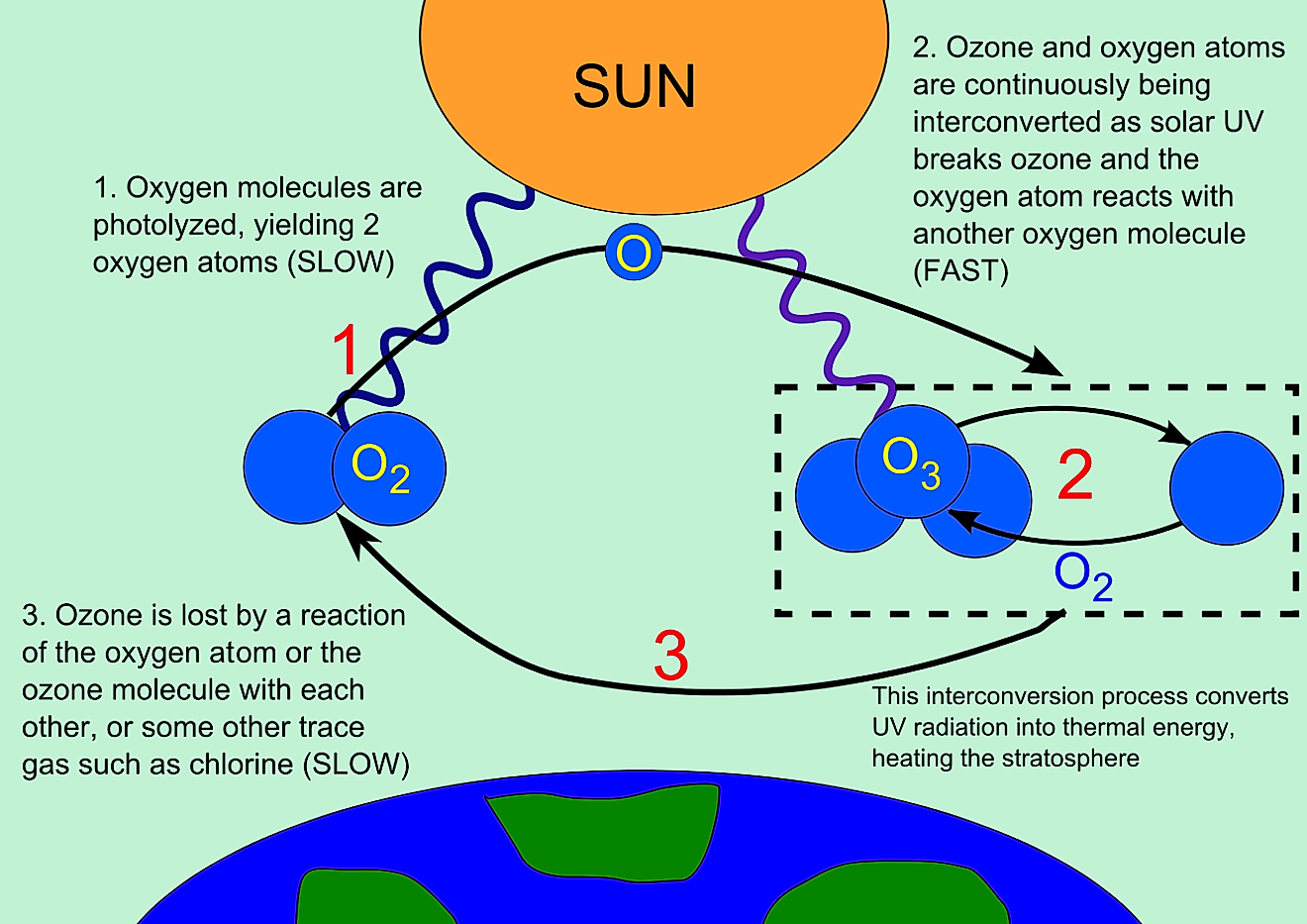
- Ozone is a molecule with a strong smell and is blue in color.
- Molecules of ozone consist of three oxygen atoms.
- Ozone was discovered by Christian Friedrich Schönbein in 1840.
Despite popular beliefs, the ozone hole is not actually a hole in the ozone layer, but a region of depleted ozone in the stratosphere over the Antarctic, which occurs at the beginning of spring in the southern hemisphere, sometime between August and October. In recent years, the ozone depletion has also been noted in the northern polar region. The phenomenon is called ozone hole.
This stratospheric ozone layer protects Earth by absorbing harmful ultraviolet light, which can damage the DNA of plants and animals on Earth.
How Do Chloroflourocarbons Affect Ozone?

It has become general knowledge that chemicals called CFCs (chloroflourocarbons) are responsible for the hole in the ozone layer - in fact, scientists estimate 80% of CFCs in the stratosphere are derived from humans, not nature. These chemicals escape from refrigeration and propellant devices and processes, such as refrigerators and air conditioners, large coolers and chillers, aerosol can propellants, blowing agents for making plastic foams, firefighting agents, and solvents used in dry cleaning and degreasing, etc. They do not easily degenerate. In fact, CFCs can linger in the lower atmosphere for decades, and their long lifespan allows some of the chemicals to reach the stratosphere. There, ultraviolet light breaks down the bond holding chlorine atoms to the CFC molecule, and the free chlorine atoms are able to participate in a number of chemical reactions, which destroy ozone.
However, contrary to popular belief, the breakdown of ozone caused by chlorine does not occur immediately. Most of the chlorine becomes part of two separate chemicals, which under normal circumstances are stable. But in polar darkness, the atmospheric conditions become unusual, and the circling stratospheric winds known as the polar vortex make the air so cold and dry that chemical reactions that ordinarily could not happen will take place.
Inactive chlorine, which has been stored in two stable chemicals, is converted to active forms like chlorine gas, and when sunlight returns the UV light breaks the bond between chlorine atoms, releasing chlorine into the air. Catalytic reactions allow single chlorine atoms to destroy thousands of ozone molecules.
When Does The Ozone Hole Grow?
The hole will grow through early spring, until temperatures warm and the polar vortex becomes weak enough to allow air from surrounding regions into the polar zone. The mixing of different air systems allows the chlorine to disperse and the ozone layer stabilizes itself until the following spring when the polar vortex picks up again.
Can The Ozone Layer Be Saved?
In 1987, as the world came to realize the impacts of CFCs on the ozone layer, the Montreal Protocol on Substances that Deplete the Ozone Layer was developed after an intense series of international meetings and negotiations, to phase out the production of ozone-depleting chemicals by 2000. The Protocol addressed CFCs, halons, carbon tetrachloride, and methyl chloroform, all of which could deplete the ozone layer under the right conditions. A series of amendments to the Protocol followed in subsequent years, and by 2005 the consumption of ozone-depleting chemicals had dropped by 90 to 95% in participating countries.
Scientists have logged images showing the ozone is recovering with more awareness and change in human behavior, with a 20% decrease in ozone depletion between 2005 and 2016. In 2019, the smallest ozone hole since 1982 was recorded over the Antarctica.
However, in 2020 the largest hole ever observed was found over the Arctic and took longer than usual to close due to atmospheric conditions. Scientists spotted a rare large hole forming in late March, assumed to be caused by low temperatures at the north pole. By April 23, it was announced the hole had closed.
The Arctic ozone hole is rare, as the northern hemisphere is not ordinarily prone to the strong and stable polar vortex conditions necessary to allow ozone-depleting chemicals to become concentrated and destroy ozone molecules. Scientists are still working to determine what made atmospheric conditions ripe for an Arctic ozone hole in 2020.
According to simulation models, it is estimated the ozone layer will be mostly recovered by 2040.