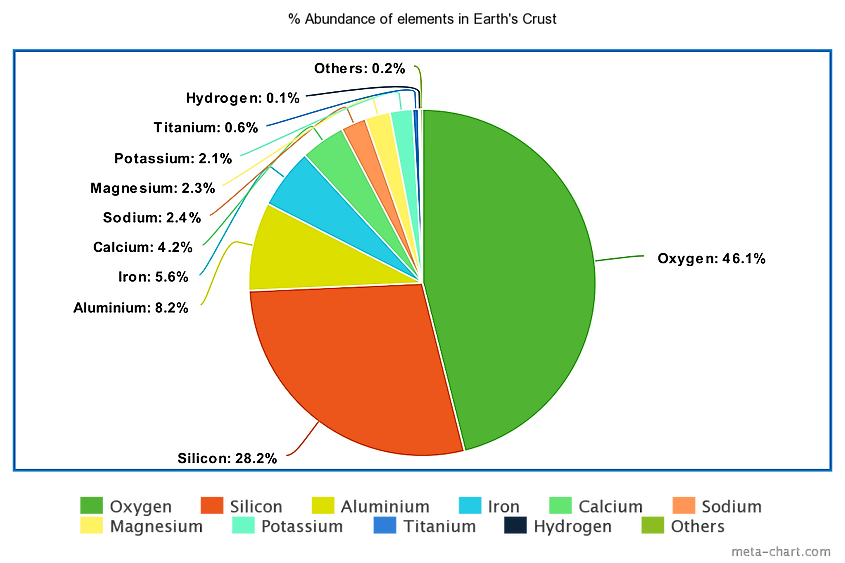
The Most Abundant Elements In The Earth's Crust
The Earth’s Crust
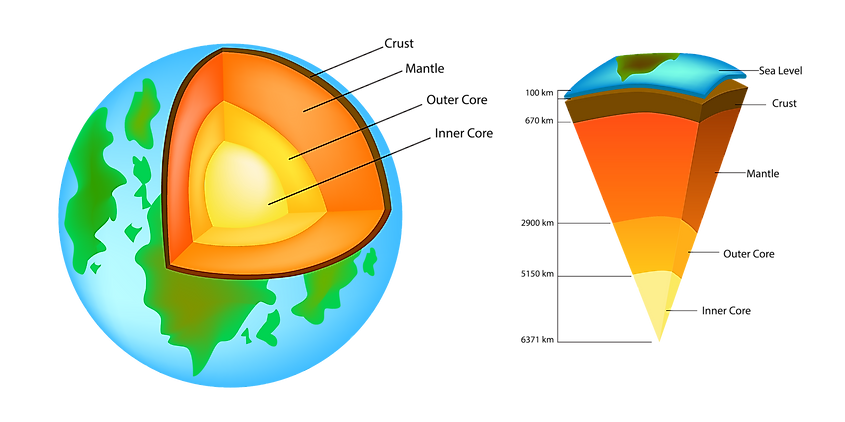
The crust is the outermost layer of a planet. The Earth is composed of a number of different elements, but the crust only makes up 1% of the Earth’s volume in a thin outer layer which encases the other parts of the inner planet including the mantle, outer core and inner core. The Earth’s crust is 25 miles, or 40 km deep and is composed of solid rocks and minerals that cooled and solidified when the Earth was young. There are many different elements which can be found in the crust. The 10 most abundant elements (as per the CRC Handbook of Chemistry and Physics, 97th edition) in the Earth's crust by % of abundance are mentioned below:For the prevalence of all elements, click here to scroll down.
10 Most Abundant Elements In Earth's Crust
| Rank | Element | Abundance (percent by weight) |
|---|---|---|
| 1 | Oxygen | 46.1% |
| 2 | Silicon | 28.2% |
| 3 | Aluminum | 8.23% |
| 4 | Iron | 5.63% |
| 5 | Calcium | 4.15% |
| 6 | Sodium | 2.36% |
| 7 | Magnesium | 2.33% |
| 8 | Potassium | 2.09% |
| 9 | Titanium | 0.565% |
| 10 | Hydrogen | 0.140% |
1. Oxygen (O)
One of the most prominent and important elements that make up the crust of the earth is Oxygen. Oxygen is the most abundant element in the Earth’s crust, at 461,000 parts per million. This means it makes up roughly 46% of the Earth’s crust. Within the universe at large, Oxygen ranks number three in abundance. Oxygen makes up 21% of the Earth’s atmosphere and 90% of the mass of water. It is arguably the most important element to life on Earth, and indeed it comprises roughly two thirds of the human body’s components. Oxygen is an element which is highly reactive and also easily combines with other elements. Because of this, oxygen is found in a large number of common compounds both on Earth and in the crust, specifically. In the Earth’s crust, there is a great deal silicate, which is formed from silicon and oxygen. Oxygen also pairs with iron to create iron ore and various iron compounds which make up much of the Earth’s crust. Liquid oxygen is highly combustible and used as a fuel, while oxygen and acetylene creates a flame hot enough for welding and metal melting. Even more than this, most organic life on earth requires oxygen for survival. It is one of the main components in most living things.
2. Silicon (Si)
As mentioned in the case of silicate, silicon is also a prominent element found in the Earth’s crust. It makes up some 28% of the crust, and can be found in a wide variety of minerals and elemental compounds, usually in conjunction with oxygen. Silicon dioxide is one of the most common of these compounds, and is composed of silicon and oxygen. Silicon dioxide is the main component of many types of hard crystalline rocks such as quartz, amethyst, opal and rock crystal. Silicon Dioxide is also what most sand is made out of, and a large part of the reason it is so commonly found in the earth’s crust. Sand is mostly made up of silicon based minerals and rocks. Silicon is also used in a variety of human made products such as most electronics, and microchips as well as glass products and bricks.
3. Aluminum (Al)
Aluminum, third on the list of most abundant elements, comprises roughly 8% of the Earth’s crust, and is actually the most abundant metal in the crust. Though it is the most commonly found metal, it is always found in compound form, never in its raw state. The most commonly found compounds are potassium aluminum sulphate, and aluminium oxide.
4. Iron (Fe)
Approximately 5% of the Earth’s crust is iron. Iron is a very important element on Earth, and it actually makes up the majority of the Earth’s core. Also, due to its abundance, it has been used by humans for thousands of years, even lending itself to the naming of an Era in the Iron Age. Though humans have developed greatly since the Iron Age, iron is still a prominently used metal in modern times. Iron and carbon combine to make steel, one of the most used metals in everything from small household items to bridges and buildings. Iron is also important to organic life. It is a key part of human blood, and is a component in chlorophyll in plants.
5. Calcium (Ca)
Calcium accounts for around 4% of the Earth’s crust. Though calcium is usually affiliated with human growth in relation to bones and development, calcium is also readily found in the Earth in various compound forms and is often found in combination with oxygen or water. Calcium carbonate is also a common compound, and can be found in a variety of rock types such as marble, chalk and limestone, as well as shells and pearls.
6. Sodium (Na)
At roughly 2.3% of the Earth’s crust, Sodium ranks number 6 on the list of most abundant elements. Like many of the elements on this list, it is never found free in nature, but rather in compound form. It is also a highly reactive element when in its isolated form. For humans, sodium is often most associated with rock salt - sodium chloride. As it is very water soluble, sodium is one of the most common dissolved elements found in the ocean, and indeed saltwater bodies often produce sodium chloride, or salt deposits especially where the body of water has dried up. Sodium is also an essential element for animals and humans, and help organic life maintain adequate fluid balance which in turn effects nerves and muscle fibres.
7. Magnesium (Mg)
Magnesium is the 7th most common element in the Earth's crust with an abundance of about 2%. The metal does not occur as a free element but in combination with other elements like oxygen, calcium, and carbon. Dolomite is an example of a mineral containing magnesium.
8. Potassium (K)
Approximately 2% of the Earth’s crust is potassium. It is not an element that is found in its solitary form in nature, but is in a number of compounds found freely within the earth. Its pure form is highly reactive to both oxygen and hydrogen, meaning it can ignite when in water or open air. Naturally, potassium can be found in potash and various minerals such as carnality, sylvite or polyhalite. The most common potassium compound is potassium chloride which is used in fertilizers and the like, and potassium carbonate which is used for soaps and certain types of glass.
9. Titanium (Ti)
Titanium can be found in minerals such as rutile, ilmenite and sphene, which can be found in the Earth’s crust. At 0.6 % of the Earth crust’s make up, it is far less abundant than the elements which hold spots one through eight on the list. Still, it is an important element and is known for being both extremely strong, and very light. Because of this it is used in a variety of ways by humans, for everything from airplanes to artificial human joints.
10. Hydrogen (H)
Hydrogen is actually the most abundant element in the known universe, but it only makes number ten with regards to elements in the Earth’s crust as it is most commonly found as a gas. Hydrogen has many compounds which are readily found on Earth both in nature and in human made uses. Hydrogen is of course a key component in water, H2O, but is also in the common compounds ammonia, methane, hydrogen peroxide and even sugar, all of which are readily used by humans.
The Earth's crust is predominated by oxygen and silicon, which constitute approximately 74.3% of its composition by weight, essential for the formation of rocks and minerals. Oxygen's prevalence is linked to its role in supporting life, while silicon's abundance is crucial for its use in technology and construction. The other elements, though less abundant, are emblematic of a phrase coined by Aristotle, "the whole is greater than the sum of its parts."
The Most Abundant Elements In The Earth's Crust
| Rank | Element | Symbol | Abundance in crust (ppm) by source |
|---|---|---|---|
| 1 | Oxygen | O | 461,000 |
| 2 | Silicon | Si | 282,000 |
| 3 | Aluminium | Al | 82,300 |
| 4 | Iron | Fe | 56,300 |
| 5 | Calcium | Ca | 41,500 |
| 6 | Sodium | Na | 23,600 |
| 7 | Magnesium | Mg | 23,300 |
| 8 | Potassium | K | 20,900 |
| 9 | Titanium | Ti | 5,650 |
| 10 | Hydrogen | H | 1,400 |
| 11 | Phosphorus | P | 1,050 |
| 12 | Manganese | Mn | 950 |
| 13 | Fluorine | F | 585 |
| 14 | Barium | Ba | 425 |
| 15 | Strontium | Sr | 370 |
| 16 | Sulfur | S | 350 |
| 17 | Carbon | C | 200 |
| 18 | Zirconium | Zr | 165 |
| 19 | Chlorine | Cl | 145 |
| 20 | Vanadium | V | 120 |
| 21 | Chromium | Cr | 102 |
| 22 | Rubidium | Rb | 90 |
| 23 | Nickel | Ni | 84 |
| 24 | Zinc | Zn | 70 |
| 25 | Copper | Cu | 60 |
| 26 | Cerium | Ce | 66.5 |
| 27 | Neodymium | Nd | 41.5 |
| 28 | Lanthanum | La | 39 |
| 29 | Yttrium | Y | 33 |
| 30 | Nitrogen | N | 19 |
| 31 | Cobalt | Co | 25 |
| 32 | Scandium | Sc | 22 |
| 33 | Lithium | Li | 20 |
| 34 | Niobium | Nb | 20 |
| 35 | Gallium | Ga | 19 |
| 36 | Lead | Pb | 14 |
| 37 | Boron | B | 10 |
| 38 | Thorium | Th | 9.6 |
| 39 | Praseodymium | Pr | 9.2 |
| 40 | Samarium | Sm | 7.05 |
| 41 | Gadolinium | Gd | 6.2 |
| 42 | Dysprosium | Dy | 5.2 |
| 43 | Erbium | Er | 3.5 |
| 44 | Ytterbium | Yb | 3.2 |
| 45 | Hafnium | Hf | 3.0 |
| 46 | Caesium | Cs | 3 |
| 47 | Beryllium | Be | 2.8 |
| 48 | Uranium | U | 2.7 |
| 49 | Bromine | Br | 2.4 |
| 50 | Tin | Sn | 2.3 |
| 51 | Europium | Eu | 2.0 |
| 52 | Arsenic | As | 1.8 |
| 53 | Tantalum | Ta | 2.0 |
| 54 | Germanium | Ge | 1.5 |
| 55 | Tungsten | W | 1.25 |
| 56 | Molybdenum | Mo | 1.2 |
| 57 | Holmium | Ho | 1.3 |
| 58 | Terbium | Tb | 1.2 |
| 59 | Thallium | Tl | 0.850 |
| 60 | Lutetium | Lu | 0.8 |
| 61 | Thulium | Tm | 0.52 |
| 62 | Iodine | I | 0.450 |
| 63 | Indium | In | 0.250 |
| 64 | Antimony | Sb | 0.2 |
| 65 | Cadmium | Cd | 0.15 |
| 66 | Mercury | Hg | 0.085 |
| 67 | Silver | Ag | 0.075 |
| 68 | Selenium | Se | 0.05 |
| 69 | Palladium | Pd | 0.015 |
| 70 | Bismuth | Bi | 0.0085 |
| 71 | Platinum | Pt | 0.005 |
| 72 | Gold | Au | 0.004 |
| 73 | Osmium | Os | 0.0015 |
| 74 | Tellurium | Te | 0.001 |
| 75 | Ruthenium | Ru | 0.001 |
| 76 | Iridium | Ir | 0.001 |
| 77 | Rhodium | Rh | 0.001 |
| 78 | Rhenium | Re | 0.0007 |